KINTARO Cells Power is responsible for creating high quality mesenchymal stem cells by incorporating an accumulation of over 50 years of Russian based technological achievements, and enhanced using advanced Japanese culturing technology. KINTARO Cells® are multipotent Bone Marrow derived Mesenchymal stem cells that have the ability to differentiate into various cells. Differently from autologous adipose derived cells, which are taken from a patient to be carried out on themselves, we use allogeneic stem cells taken from young healthy donors at their 20s. Autologous cells are still widely used in Japan; however, the mainstream use of cells therapy has already begun to shift to allogeneic cells.
Only 1 in 100,000 stem cells in bone marrow has the same properties as ours.
KINTARO Cells® is CD34 (-) bone marrow derived mesenchymal stem cell, which is multipotent and capable of differentiating into various cells. All parts which need regeneration (bones and muscles, blood vessels, organs, skin and more) can be regenerated using KINTARO Cells®. KINTARO Cells® are collected from young healthy donors in their 20s and cultivated with our innovative biotechnology.
Clinical trial data of mesenchymal stem cells in the world (specification frequency by origin):
other adipose derived mesenchymal stem cells, umbilical cord blood derived mesenchymal stem cells, bone marrow derived MSCs.
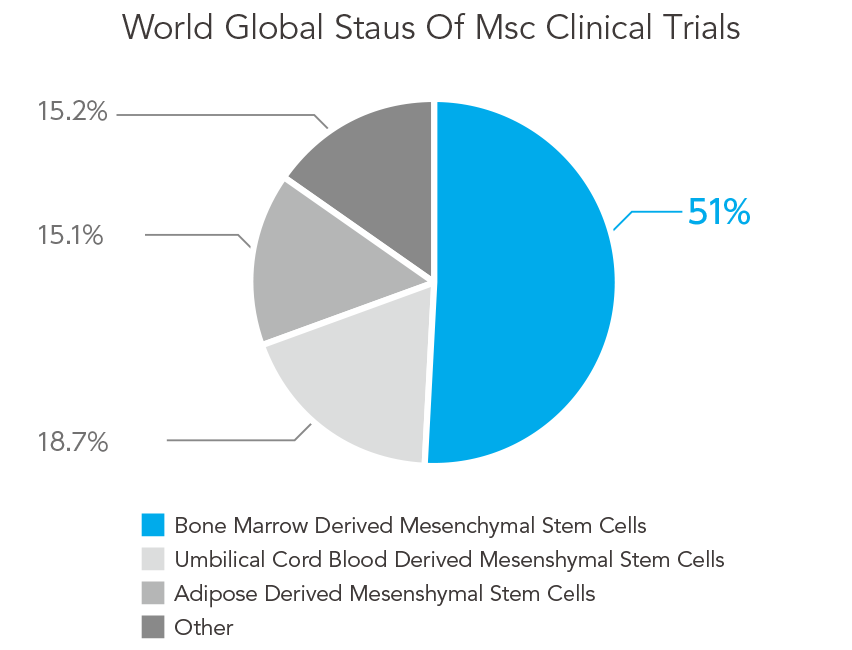
Reference: Clinical trials.gov 2015
Allogeneic Human Bone Marrow derived Mesenchymal stem cells are cultivated using our patented* culturing technology. Advanced biotechnology skills are required for this cell culturing. The cultured cells that meet our own strict quality standards will become “KINTARO Cells®“.
* Japanese Patent No. 6153653