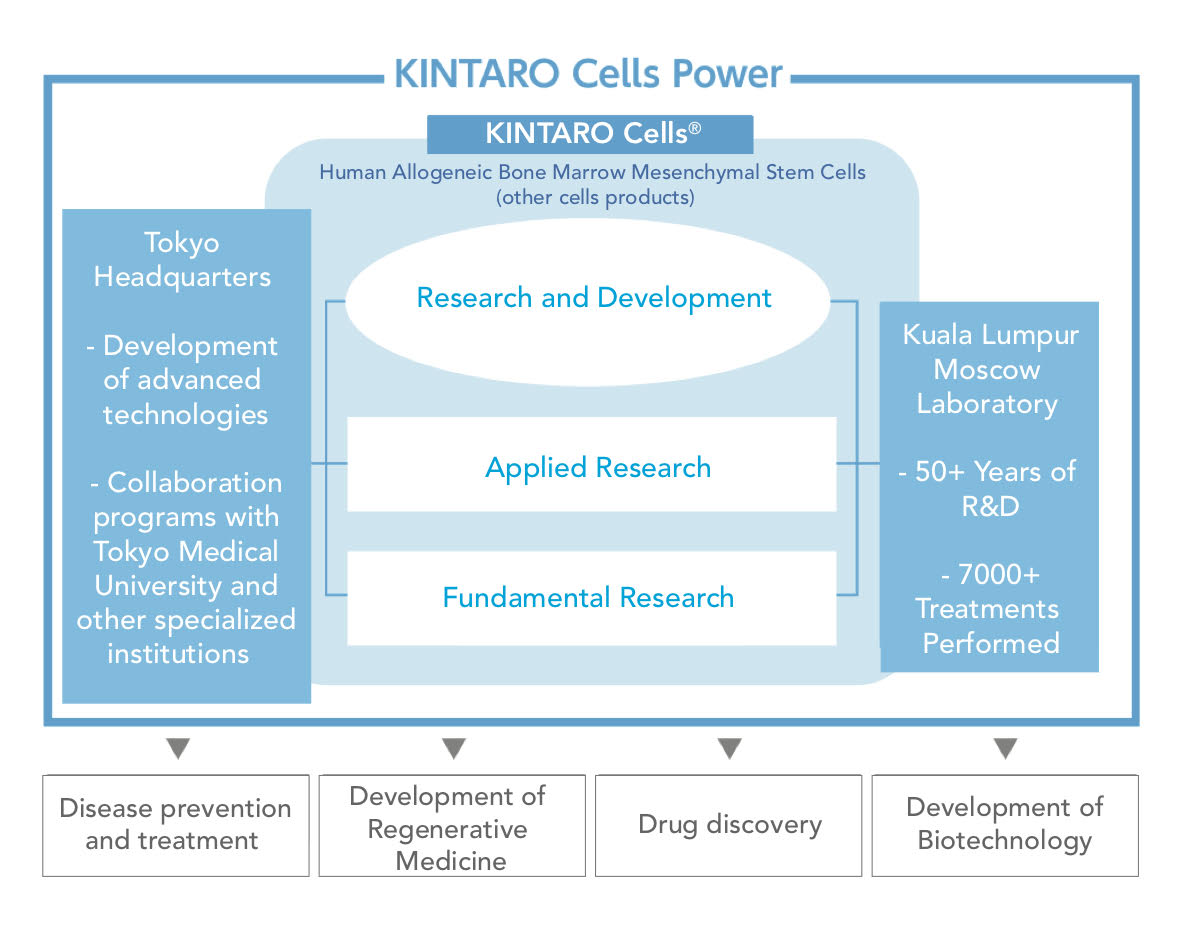
The manufacturing and development of “KINTARO Cells®” is an amalgamation between advanced biotechnological achievements of Russia and Japan. Our laboratories have accumulated over 50 years of research and development experience in addition to more than 7,000 clinical trials and treatments. KINTARO Cells® takes advantage of existing advanced biotechnological applications in Japan with continued collaborative research with Tokyo Medical University, which will be published at the end of 2018. In knowing the strengths of KINTARO Cells®, basic research was established with applied research commencing shortly after. The aim of KINTARO Cells® is to contribute to society, through prolonging the healthy lifespan of more people, development of regenerative medicine in the future, drug discovery, and the development of basic science.
Kintaro Cells Power continues to progress business development not only in Asia but also around the world. As a result, we are planning establish new manufacturing facilities overseas that comply to the Good Manufacturing Practice (GMP) standard for production control and quality control of pharmaceuticals and quasi-drugs overseas. In Japan, we plan to promote drug discovery in cooperation with organizations meeting the GCTP (Good Gene, Cellular, and Tissue – based Products Manufacturing Practice) standards for practices that include production control and quality control of regenerative medicine. Furthermore, in order to reduce manufacturing and labor cost, we plan to implement fully automated cultivation. By taking the human factor out of the equation, we can greatly reduce the risks of contamination, significantly save on manual labor costs, and dramatically shorten time on local production setup.
Our first goal is to design a semi-automated product, which will be operated by a trained technician through microscope and simple control interface. We aim to achieve this goal within the next 1~3 years. The first year will involve taking designs, patents, and organizing the technical development team.
The equipment will be designed so that it is easily operated by anyone and can avoid the requirement of a CPC laboratory with immense installation and running costs. Our goal is to expand this product into the biotechnology market.
This stage consists of the development of a self-automated cultivation device. AI software and the upgrade to advanced equipment that can perform automatic cell analysis. With fully automatic control by AI, “KINTARO Cells®” will be manufactured under the best conditions without any human error.
The Research team led by Professor Kazuma Oyashiki from Tokyo Medical University specializing on “Cellular Therapeutic Research and Development Course” and a research staff from Kintaro Cells Power, conducted a collaborative research on “KINTARO Cells®”. The subject of the research included analyzing the potency of “KINTARO Cells®” collected from a young donor in his twenties and comparing it to other stem cells.
Together with the research team at Tokyo Medical University, our Medical Director was present to ensure the project was heading in the right direction.
The results of the detailed analysis of the difference between KINTARO Cells® from younger donors and elderly stem cells will be summarized in a research paper and will be published in a medical journal in 2019.
