The Medical Radiological Research Center is established in Obninsk, Russia
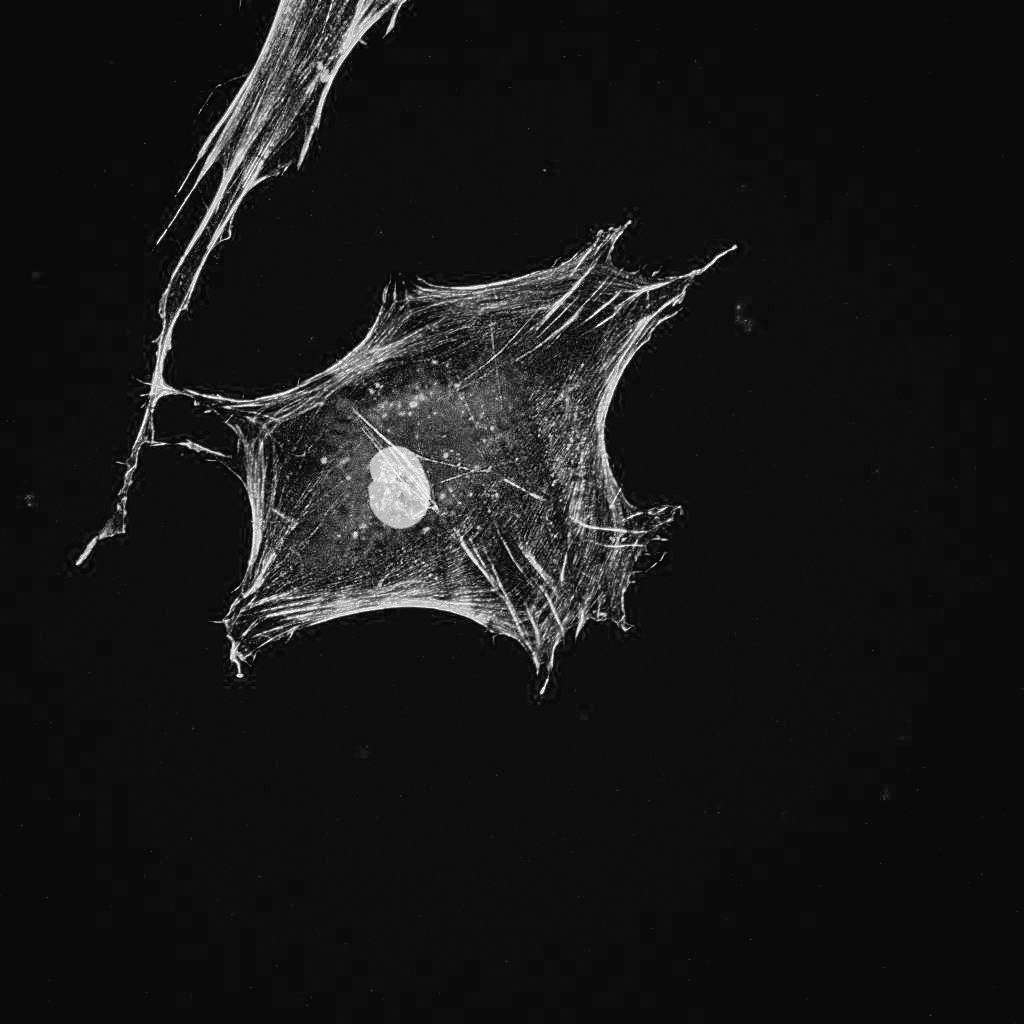
Dr. Alexander Yakovlevich Friedenstein discovers bone marrow-derived mesenchymal stem cells.
Dr. Anatoly Konoplyannikov starts the first clinical trial of bone marrow-derived mesenchymal stem cells at the Russian Radiation Research Institute in the Immune Cell Therapy Department. (To date, a total of over 7,000 procedures have been performed.)
CEO Alexei Gladkov receives treatment for bone marrow-derived mesenchymal stem cells at a medical institution in Russia. After recognizing the effect of the treatment, he develops various approaches to spreading this regenerative technology to the world.
Alexei Gladkov establishes AV Cells, which is the predecessor of Kintaro Cells Power Co., Ltd. in Singapore. Then he transfers technology of mesenchymal stem cells from Russia Radiation Research Institute to the new company, and starts the research and development of bone marrow-derived mesenchymal stem cells with innovative technologies.
AV Cells Laboratory in Novena, Singapore is established. Full-scale research and development of bone marrow-derived mesenchymal stem cells is commenced.
In Japan, the ‘Regenerative Medicine Safety Assurance Law’ becomes a new standard of law. It guarantees the safety of program sponsorship of regenerative medicine and cell culture organizations, by providing them with a legislative basis. At the same time, in order to respond to the practical use of regenerative medicine, amendments are being made to the Pharmaceutical Affairs Law, establishing a system for approving the characteristics of regenerative medicine products. Kintaro Cells Power Co., Ltd. is established by Alexei Gladkov to integrate the production of bone marrow-derived mesenchymal stem cells in Russia with high cultivation technology in Japan, thus initiating the research and development of such cells.
Branches of Kintaro Cells Power Co., Ltd. are established in Asian countries including Thailand, the Philippines, and Indonesia. A subsidiary in Hanoi and Saigon, Vietnam, is established as well. At a medical-related international event in Hanoi, Vietnam, Kintaro Cells Power is announced under the brand name «KINTARO Cells®»* for the first time. An application for six patents is filed, which includes: Culturing Method, Single Type Stem Cell, Medium Culturing Method, Product Culture Medium, Method of Stem Cell Activation, and Activated Stem Cells.*The first product of the current «KINTARO Cells Basic»
Kintaro Cells Power Corporation starts collaborative research with Dr. Kazuma Oyashiki from Tokyo Medical University and his team, who are accomplishing research and development in the field of cell therapy. While using «KINTARO Cells®» as the research object, the differences between young and elderly stem cells are analyzed in detail. The trademarks are registered in Japan, Korea, Russia, Hong Kong, and Taiwan. Patents are obtained in Japan for the «Stem Cell Culturing Method».
Two patents are acquired in Japan: one for the «Product Culture Medium» — culture production fluid manufacture method, — and another for the «Method for Activated Stem Cells.» A branch office in Malaysia is established. Search for an institutional investor begins, and an affiliate company is established to conduct drug development. Business services for foreign patients are being expanded through cooperation with foreign branches. On August 4, 2018, our collaborative research team at Japan Human Cell Society officially announces the results of the study and analysis of the differences between young and aged stem cells (while using «KINTARO Cells®» as study objects).
A new research laboratory is established in Malaysia. Projects aimed at developing our business in India, Cambodia, Myanmar, and Middle Eastern countries are promoted. «KINTARO Cells Plus»* is released. The Otaru Oshoro Laboratory, which has a sterile room for research on various cells, is opened in Oshoro, Otaru City, Hokkaido.The world’s first cell shrine is built on its premises. *A product with extended applications of «Kintaro Cells Basic»
The results of the joint research project on «KINTARO Cells®» with Tokyo Medical University are published in a scientific journal, «HUMAN CELL».
The Japanese patent for «BM MSC-Supernatant Solution» is acqiesed. Every year, we hold the «Kintaro Sakura Festival» in May, the «Reitaisai» in October, and the «Yukiakari no Michi Festival» in February.
KINTARO Cells Power hosts a performance event «Light of Kintaro, The Power of The Future» on stage in Tokyo In February. The Otaru performance of the play is held in August.
The Kyoto performance of «Light of Kintaro: The Power of The Future» is held in March. The «KINTARO Fully Automated Robotization Factory» project, which fuses AI technology and robotics, is underway. In July, the Japanese patent for «Stem Cell Transportation Method» is acquired.